#2: The PrEPisode
COMMUNITY VOICE: Damon Jacobs |. HEALTHCARE EXPERTS: Oni Blackstock MD MHS, Robert Pitts MD
SHOW NOTES
Some definitions
PrEP: pre-exposure prophylaxis - referring to a daily medication taken to prevent the acquisition of HIV
PEP: post-exposure prophylaxis - referring to a combination of medications taken after a potential exposure to HIV in order to reduce the likelihood of transmission
Who is a candidate for PrEP?
People who have condom-less anal sex
People who have sex partners they don't know very well or whose partners know they have HIV
People who come from communities that have a greater burden of HIV because of less access to care and other systemic barriers
Anyone who has had a bacterial sexually transmitted infection, like gonorrhea, chlamydia, or syphilis in the past six months
Why is PrEP special?
PrEP is a powerful form of prevention that allows people to have autonomy and make decisions about their own sexual health
It can serve as a gateway into long-term primary care (which we here at QHP are big fans of!)
PrEP is not only a medication that prevents HIV, but it's also a very emotionally significant drug for many. For those who feel the legacy of the AIDS crisis of the 1980s and 1990s, having an HIV prevention drug can provide agency and control.
In the history of the AIDS epidemic, GBQ men were often unwelcome and vilified in medicine. The intention is not to say no one else is ignored. The LGBTQ+ community as a whole is often ignored in health care. As Damon himself recognizes, exclusion from the medical community is often acutely felt by those who hold marginalized identities.
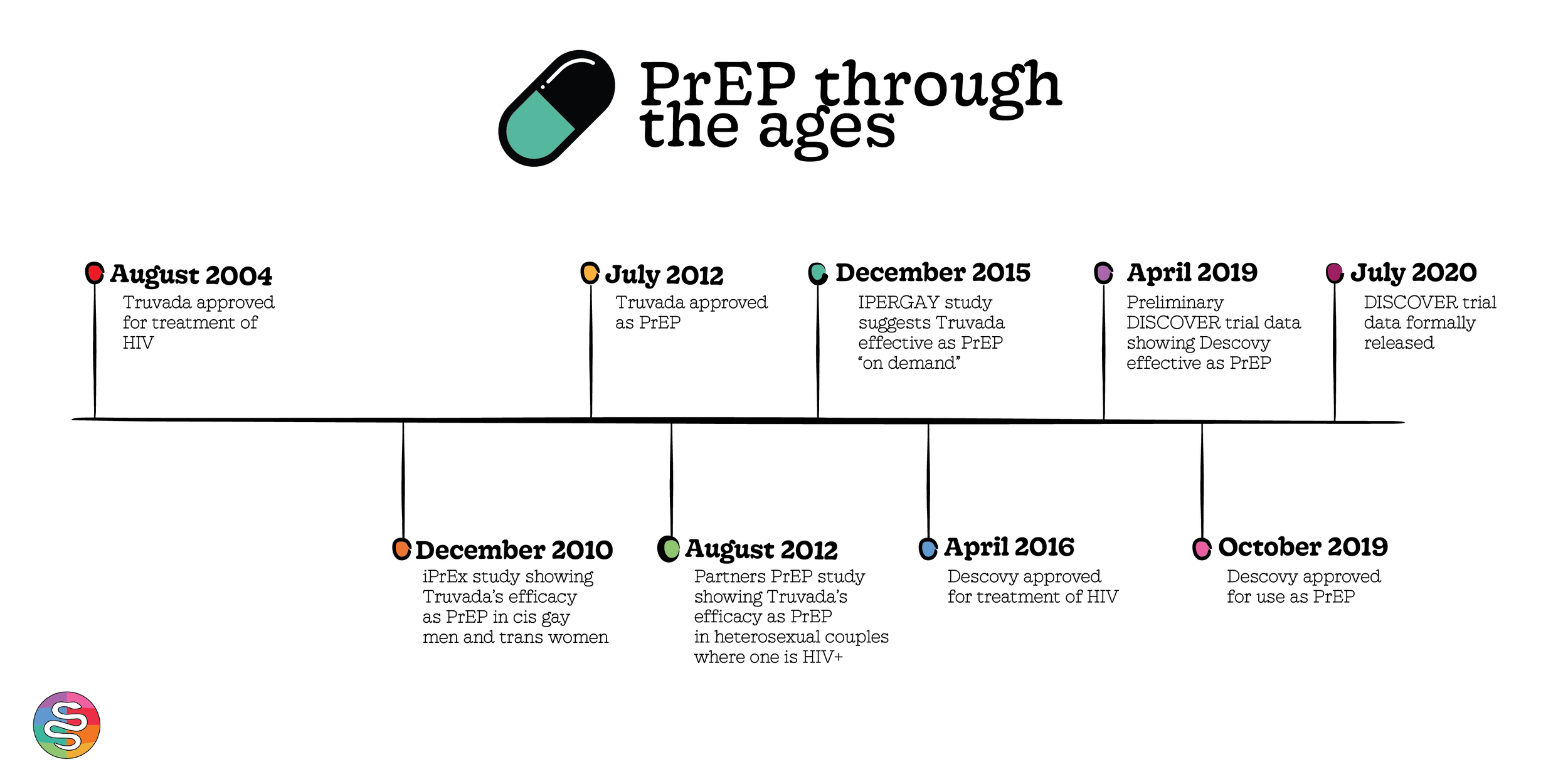
A brief PrEP timeline
In 2012, Truvada (otherwise known as tenofovir/emtricitabine/TDF) - a medication previously used to treat HIV - was approved by the FDA for PrEP, or to prevent HIV.
It became apparent that Truvada as PrEP worked. The first big study looking at Truvada showed that daily PrEP lowered the risk of getting HIV by 92% if exposed.
Slowly but surely, the queer community got on board (with the help of groups like PrEP Facts). More and more people began using PrEP to prevent HIV transmission.
2019: a scientific trial called the DISCOVER trial published preliminary data
It investigated the possibility of a second PrEP medication, another HIV medication called Descovy (otherwise known as tenofovir/emtricitabine/TAF)
It also suggested that Descovy may have a better side effect profile
Based on the preliminary data, the FDA approved Descovy as a second PrEP option. Some folks started getting switched from Truvada to Descovy (with or without their consent).
At which point many people started wondering: which PrEP medication to opt for? Which is the better option?
Truvada vs. Descovy: the scientific data
When Truvada came onto the scene as PrEP, it did so with multiple large studies of high quality evidence and in multiple populations
iPrEx: a study that put Truvada on the map. Studied side effects and efficacy at HIV prevention in men who have sex with men as well as transgender women.
Partners PrEP: showed that Truvada was effective at HIV prevention within heterosexual couples where one partner is HIV+
Descovy, in turn, so far has less scientific literature discussing its efficacy as PrEP in a variety of populations
Basically, all we have is the DISCOVER trial - which only looks at cisgender men and some trans women
And note: up until 2020 (a year after FDA approval) the DISCOVER trial had not officially released its data, just a preliminary abstract
It’s unusual that Descovy was such a popular and widely-used option before the data was officially published – before healthcare providers could read the data for themselves and help their patients make informed decisions
In addition to the above differences in how the drug came to the market, Truvada has an additional study (called IPERGAY - you can’t make this stuff up)
This study demonstrates Truvada’s efficacy as PrEP “on demand” – meaning, taken in the days right before and right after a sexual encounter
Descovy, in turn, has no such data supporting its use as PrEP on demand
Truvada vs. Descovy: side effects
Truvada
Abdominal discomfort: observed in roughly 1 out of 5 people. Usually goes away on its own after two to six weeks on medication
Changes in kidney function (as measured via a substance called creatinine), largely reversible
Changes in bone density - also reversible once taken off the medication
Descovy
Based off of what we know about Descovy as HIV treatment, it can increase cholesterol, blood sugar levels, or weight gain, risk factors for the development of heart attack and stroke.
Unclear if these effects will be seen or will have impact on people’s health in the long-term when Descovy is used as PrEP given that the drug is dosed differently in this context.
The DISCOVER data also suggests that there may be fewer kidney and bone side effects -
So who may benefit from Descovy as PrEP?
As of right now, the medication is approved in cisgender men who have sex with men. It is not approved for folks with vaginas, since that group was not studied in the DISCOVER trial.
Ultimately, the decision is an individualized one that depends on personal medical history and preferences. For example: for folks with kidney disease, Descovy may be a better option.
Long story short: it depends. Bring it up with your primary care provider! (And if you don’t feel comfortable bringing it up, we encourage you to find a care provider with whom you do feel comfortable – all while acknowledging that this is likely far easier said than done.)
A generic PrEP option
Truvada’s patent expired in 2019, at which point it became generic. How does having two PrEP options – one brand name, one generic – change the HIV prevention landscape?
The good
On face value, having a generic PrEP option seems like a good thing since it will increase financial access.
Additionally, having two options gives folks agency, which may increase PrEP uptake
However…
Having a fancy brand name option (Descovy) may stigmatize the generic option (Truvada). This is tricky for folks who only have generic options available to them.
One drawback is that the Gilead co-pay assistance programs will not pay co-pays so there may be some back and forth with providers, insurance and pharmacies as this transition occurs. However, there should always be an option to have PrEP covered.
TRANSCRIPT
Damon: The problem is the decision has often not been made by the patient or the consumer. The problem is, these decisions are being made prescriptively by a doctor – without talking to the consumer – without consulting or involving the consumer – thereby diminishing their sense of agency. Which is the opposite of what PrEP is all about psychologically.
Sam: Welcome to Queer Health Podcast, a podcast about health for sexual and gender minorities. My name is Sam, I'm in residency training in New York, and I use he/him pronouns.
Gaby: I'm Gaby. My pronouns are she/her - and I have the same job title.
Richard: And I am Richard Greene, the medical director at the Pride Health Center at Bellevue hospital in New York City. And my pronouns are he and him.
Sam: We just heard from Damon Jacobs about the controversy surrounding the two drugs that people can take for pre-exposure prophylaxis (or PrEP) against HIV.
Richard: Damon is a therapist and founder of the very popular Facebook group PrEP facts.
Sam: PrEP stands for preexposure prophylaxis. Let's break that down. “Pre-exposure” – fairly straightforward, “before you're exposed”. “Prophylaxis” means a way to prevent something. So together, pre-exposure prophylaxis means a way to prevent something. And in this case, we're talking specifically about exposure to HIV, the virus that causes AIDS.
Richard: As we mentioned, PrEP is for people who want to prevent the transmission of HIV. This can mean people who have condom-less anal sex, people who have sex partners, they don't know very well or whose partners know they have HIV. It can also be for people who come from communities that have a greater burden of HIV because of less access to care and other systemic barriers. And finally, for anyone who has had a bacterial sexually transmitted infection, like gonorrhea, chlamydia, or syphilis in the past six months.
Sam: And just for clarity, we should say don't confuse PrEP with PEP – or post exposure prophylaxis, which is three medications you can take if you think you've been exposed to HIV.
Gaby: So again PrEP for HIV is something that someone who is HIV negative takes that stops HIV from infecting them if they become exposed to the virus. That sounds pretty simple but there's actually a lot to the PrEP story.
Sam: On today's episode, we'll spend a little bit of time talking about PrEP basics, but we'll spend a lot more time talking about the two drugs that as of summer 2020 can be taken as PrEP for HIV.
Richard: In short, this episode is for folks who are interested in preventing HIV.
Gaby: It is as simple as that.
[TRANSITION MUSIC]
Okay. So when we defined PrEP at the beginning of this episode, we talked about it. Like it was a medication, just like a blood pressure pill or something you might take to fix a headache.
Sam: But Damon spoke about PrEP as something more than just an HIV prevention medication.
Gaby: Yeah. He actually used a pretty distinct phrase to talk about PrEP.
Damon: It is a biomedical intervention that gives the consumer a sense of agency and control that we typically don't have, especially if we are HIV negative or not coping with a chronic disease or illness. The sense of being able to connect with healthcare workers and learn healthcare systems and build relationships that can last throughout the lifetime are all advantages that PrEP offers.
Gaby: So to understand the context around this, to understand why PrEP is so special, it's important to take a step back and go for a little history lesson in HIV prevention. I'll leave it to Professor Dubin to start the class off.
Sam: In 2012, the FDA approved a drug name, Truvada as the first ever form of PrEP for HIV. Truvada had previously been used as part of a drug regimen to treat HIV.
Gaby: So this medication we're talking about, called Truvada, was actually made up of two drugs called tenofovir and emtricitabine. These actually weren't new drugs; they actually have a long track record of being used to keep HIV under control. But now all of a sudden in 2012, they're being used in folks who did not have HIV to prevent getting it, rather than treating it.
Richard: Right. And this was a HUGE deal.
Sam: The very idea of PrEP at all, that you could take a pill and it would lower your risk of getting HIV was huge. But the other big deal was the actual efficacy of the drug. The first big study showed that when taken daily PrEP lowered the risk of getting HIV by 92%, if someone was exposed.
Gaby: So putting all the fancy statistics aside, basically what we're saying is PrEP works.
Richard: The drug seemed effective. In fact, it almost seemed too good to be true. Naturally, queer folks had a lot of questions. This is where community members like Damon really stepped up and began to crowdsource education and information.
Damon: I run a group on Facebook – an international group. We have 21,000 members, and we get about 5,000 visits per day. The group is called PrEP facts. So the emphasis is on facts - F-A-C-T-S facts – rethinking HIV prevention and sex.
Sam: Thanks to community advocates like Damon, more and more folks we're learning about PrEP and getting prescriptions for it.
Gaby: But the scene changed in 2019 when a scientific trial called the DISCOVER trial investigated the possibility of a second PrEP medication, a medication called Descovy.
Sam: Like Truvada, Descovy was a drug that had previously been approved as treatment for HIV. But unlike Truvada, Descovy was thought to have a more favorable side effect profile compared to Truvada. After reviewing the result of the DISCOVER trial Descovy was approved by the FDA for use as PrEP for HIV.
Gaby: So with the approval of a second option, PrEP, this treatment that so many had a good understanding of, that so many relied on to keep them safe, became much less straightforward.
Richard: And that ambiguity was incredibly stressful for patients because PrEP wasn't just an antiviral pill. It was a medication with a very complicated psychology attached to it.
Damon: PrEP for me is not only a drug that prevents HIV, but it's also a very emotional drug in a healthy way. In terms of the way it helps me cope with the impact of the AIDS crisis in my life as someone who uses PrEP himself and as someone who has survived the AIDS crisis and lost so many loved ones to that era. I started taking it in 2011 as a way to regain agency and to be able to deal with some of the emotional impact of the AIDS crisis.
Richard: It didn't help that in some places, clinicians were switching people over from Truvada to Descovy without discussing it with their patients.
Damon: My hope was that PrEP would be the means by which we could reestablish relationship, trust, and rapport with medical professionals. When doctors preempt choice and consent, they are destroying the rapport and the relationship that we have been trying to build especially in communities that traditionally and historically have reasons to mistrust the medical system, specifically people of color, and specifically gay men who historically have been ignored and or abused by the medical system.
Gaby: So in this way, Descovy’s approval served as a reminder of the tensions and trauma that can exist between healthcare providers and their patients. Now, these tensions and trauma exist elsewhere in medicine, but part of the reason Descovy’s approval caused such a stir was because it threw a real curve ball at the medical community. There were many that didn't know what to do with this new medication, all of the data that came with it.
Sam: For this reason, we turned to someone who is a medical expert on the subject, Dr. Oni Blackstock. Dr. Blackstock is a primary care doctor and an HIV specialist. who during our interview served as the assistant commissioner for the New York city health Department's Bureau of HIV. And during her tenure there, New York's HIV numbers have continued to reach historic lows and part due to interventions like PrEP.
Gaby: And one of the first things Dr. Blackstock did was highlight that Descovy and Truvada have had different levels of attention in terms of the scientific research and literature.
Dr. Blackstock: We have lots of data on Truvada as PrEP and there've been a number of studies that show that it is generally very safe. We only have the DISCOVER clinical trial for Descovy for PrEP. So we just have that trial.
Gaby: Naturally, we wanted to hear more about what these Truvada trials actually entailed.
Dr. Blackstock: so when Truvada was approved by the FDA in 2012, there were tw large clinical trials that were used as the evidence for its approval. one of those was the iPrEx study which focused on large numbers of men who have sex with men in a smaller number of women of trans experience. And then there was also the Partners PrEP study which was a study looking at heterosexual couples where one person was HIV negative and the other person was HIV positive. So we had two large clinical trials and several different populations.
Sam: To recap: when Truvada came onto the scene as PrEP, it did so with multiple large studies of high quality evidence and in multiple populations, including the studies that Dr. Blackstock mentioned.
Gaby: So the fact that Truvada had been studied in multiple populations is a subtle but really important point. It's actually the reason we know that Truvada works for men who have sex with men, trans women, cisgender women, straight folks, and also IV drug users. So the study population actually tells us who the drug is effective in.
Sam: But what did Descovy’s PrEP debut look like in terms of data and studies?
Gaby: For that answer, we turn to Dr. Robert Pitts, another medical expert on the subject, and I'll let him introduce himself.
Dr. Pitts: My name is Robert Pitts. I'm in the department of Internal Medicine, here Bellevue as well as NYU. And then I'm also in the division of Infectious Diseases and Immunology. I'm an assistant professor and one of the hats that I wear – the largest hat that I wear, the hat that I'm most proud of – is being the associate director of the LGBTQ Pride Center here at Bellevue.
Sam: He goes on to talk about the DISCOVER trial. Remember, that's the trial we mentioned before the trial that put Descovy on the map as a PrEP option.
Dr. Pitts: The obvious data is from the DISCOVER trial. and so I really want to, to understand why that data hasn't been published. What are the criticisms? What are the remarks are – what are they trying to fix in order to get that data published.
Gaby: Wait, so the data cited for Descovy as PrEP isn't actually published?!
Sam: Well, when we initially recorded this, it wasn't. But in July, 2020, the trial was published in a big medical journal called the Lancet. But this happened in the summer of 2020, and Descovy had been on the scene for a long time before that.
Richard: To clarify a bit, there are two processes going on here. For a new drug to be approved for a new indication like PrEP, the information or data about how safe it is and how well it works, needs to be presented to the FDA for approval. This is a rigorous scientific review. The publication of that data makes that information then available to experts throughout the medical community to digest and discuss. And then guidelines for how to use or when not to use these medications are developed by health organizations.
Gaby: Okay. So what I'm hearing is that even though Descovy’s data is now published, it's a little unusual that the drug was put into such wide use without having the data be widely available in the first place. And Dr. Pitts actually agreed
Dr. Pitts: It's quite alarming that we've shifted or approved Descovy as being equal to Truvada for prevention when a study - which is a landmark study - hasn’t been published.
Gaby: So just to sum it up before Truvada got approved for PrEP in 2012, we had robust data, two large trials with multiple populations of folks and then cut to 2019, when for Descovy we have one unpublished trial, the DISCOVER trial, which is studying almost entirely cisgender men.
Richard: Yes. And exactly because of who Descovy was studied in, I asked Dr. Blackstock how we think about just go be for other folks who take PrEP, meaning trans nonbinary folks, cisgender women, and other folks.
Dr. Blackstock: Right. Right. So, so, such an important question. So we know that Descovy is not indicated for people at risk for HIV who have vaginas. Gilead actually had sought approval for Descovy for cisgender women based on the data that they had from the DISCOVER trial, which was among MSM and trans women – and so wanted to use that data and extrapolate it, to cisgender women, which is concerning cause there should be a separate clinical trial for a different population.
Gaby: What can be confusing is how people have this conception that Truvada and discovery are equally effective. But the caveat here is that we only have data on cisgender men having sex with men and possibly transgender women. And so those are the only populations we can really draw conclusions from, from this preliminary data.
Dr. Blackstock: Gilead had claimed it would have required a lot more resources, like too many resources to enroll, a sufficient number of, cisgender women in particular in the trial, and to closely monitor their adherence.
Sam: It's worth noting that Gilead has stated that they are doing followup studies to make the data more robust.
Gaby: And to Dr. Blackstock, the structure of these future followup studies – we're talking here who is included and how those folks are being recruited – that's going to be the key.
Dr. Blackstock: And we really have to hold Gilead totally doing this study and ensuring the study design and recruitment implementation are all really informed by community engagement. And I think, they, the focus of the second study is on cisgender women, I think, but also definitely including non-binary and trans folks with vaginas is also incredibly important. We cannot continue to lump trans women with men who have sex with men.
Sam: JK Rowling, this episode's for you. This has been a lot of data diving, so let's summarize. Descovy is the new kid on the block for PrEP for HIV.
Gaby: But it's only been studied in cisgender men in the context of HIV acquisition during sex. In contrast, we have seven years of safety data on Truvada as PrEP for HIV.
Sam: Both our experts highlighted that they want to see Descovy as PrEP studied more and trans women folks with vaginas and nonbinary people specifically.
Gaby: Those who are listening might be confused. If Descovy’s still got to prove its efficacy outside of cis men who have sex with men and the data documenting its safety isn't as extensive as Truvada - then why are people switching folks over to the newer drug?
Sam: To answer this, let's get into the side effects of both drugs.
Richard: The major side effects for Truvada as PrEP are possibly diarrhea and abdominal discomfort in like one out of five people, which mostly goes away on its own after about two to six weeks on the medication.
Sam: Richard, what about kidneys? Because Damon brought that up immediately –
Damon: So one of the rumors in the – in the internet sphere, is that if you take Truvada, you're going to damage your kidneys
Richard: Rarely, there can be a slight increase in a substance in your blood called creatinine. This measures how well your kidneys are filtering. But in general, it goes away when the medication is stopped and isn't really seen that much in most people. And then finally there have been some changes seen in the density of your bones. Mostly seen in people who were using Truvada as treatment for their HIV over many years. And when it's been seen in people taking PrEP has largely gone away when they stop the medication.
Gaby: So overall, what I'm hearing is Truvada is a pretty safe drug with largely reversible side effects. Now to compare, let's talk about Descovy.
Sam: The majority of what we know about discovery side effects comes from looking at it as HIV treatment, not as PrEP. In the setting of HIV treatment, we know it causes changes to your cholesterol, elevated blood sugar levels, and a little bit of weight gain.
Gaby: So, what you're saying is you don't have a sense of whether these will be side effects for Descovy as PrEP.
Sam: Right because the dosing and frequency is different. There is some information on Descovy as PrEP that comes from the DISCOVER trial. And that data suggests that discovery may have better bone and kidney safety when compared to Truvada.
Richard: Remember that the side effects are not the whole picture. When we talk about a medication, we still have to consider how effective the drug is overall and in whom it's been shown to be effective.
Gaby: This gets us right back to what Dr. Blackstock was discussing earlier the need for more trials with a more diverse group of participants.
Sam: But for many folks who consume PrEP, recent conversations have been centered around just side effects for the most part. And focusing specifically on the perception that Descovy may be safer.
Richard: in fact, Truvada and discovery are both incredibly effective medications. Part of why people get so up in arms about the side effects with both drugs being incredibly effective is that when you take Truvada for PrEP, you're taking a medication that you don't need to prevent something from happening that might not otherwise happen. So side effects are less tolerated by people when they're considering these medications.
Sam: Damon talked about how people have really focused on the kidney side effects for the medications.
Damon: So when Descovy came around, the selling point was that Descovy doesn't affect your kidneys the way Truvada does. And for consumers who didn't know of the data and didn't know the facts about Truvada were like, “Yay, sign me up for this because you know, I don't want to do anything to put my kidneys at risk. I want protection from HIV and I want to protect my kidneys at the same time. So Descovy is the best way to do that”. That is the bill of goods they were sold.
Richard: We also asked Dr. Blackstock about where misperceptions around the side effects and safety of Truvada and Descovy came from.
Dr. Blackstock: There are a number of different class action lawsuits against Gilead because of potential delays in bringing Descovy online – and delays that people thought were associated with the fact that there are potential bone and kidney side effects that Truvada has, particularly in people who are living with HIV. And that Descovy would have been a safer alternative. But that the maker wanted to really prolong the patent life of Descovy and so had Truvada around much longer.
Sam: To say it again, a lawsuit accuses Gilead - the drug maker - of putting people at risk for Truvada side effects by not making Descovy more accessible earlier.
Dr. Blackstock: What ended up happening is there were all these different commercials on social media as well as on TV, which made people concerned about whether this medication they were taking could be potentially harmful to their kidneys or bones.
Richard: The takeaway is that because of this lawsuit and commercials on social media about the side effects of Truvada, people started getting up in arms and this created a fear about Truvada and a desire for a new medication that had fewer side effects.
Gaby: So for now, just know that ongoing legal battles led to confusion around the perception of safety for both Truvada and Descovy.
Sam: Damon also pointed out that in the PrEP Facts Facebook group, another narrative emerged that pointed to why folks might be getting switched to Descovy for prep. He talked about how providers in the group were seeing increased communication from Gilead about switching PrEP consumers to Descovy.
Damon: it started to make complete sense to me on January 13th of 2020 because that was the day that the CEO of Gilead named Daniel O'Day publicly stated that the reason they are trying to switch people from Truvada to Descovy is because of Gilead's LOE, which stands for loss of exclusivity, which means that they're losing the exclusive patent on Truvada in September of 2020. And their goal is to switch 45% of all PrEP consumers in the United States from Truvada to Descovy by the end of 2020. This is not hidden. This is all public. This is completely public information and it's been reemphasized on several stockholder calls.
Gaby: At this point, both Damon and Dr. Blackstock have spoken to how those who wanted to take PrEP – and those who wanted to prescribe PrEP - were likely exposed to misleading or confusing information. Information that made Truvada look less safe than Descovy.
Sam: So that leaves the question: when is it appropriate to utilize the newer option?
Dr. Pitts: If someone is older in their sixties, seventies, or eighties, has a history of bone mineral density disorder or has a history of chronic renal disease, then I will probably start to push a Descovy over Truvada just purely in terms of safety.
Richard: What Dr. Pitts is summarizing is how folks with different medical conditions might be the people who Descovy is safer for in the context of their specific health profile.
Gaby: And in fact for one of his patients who had a medical condition having a second PrEP option was really empowering.
Dr. Pitts: ...an MSM who had recently lost his partner and was ready to mingle again, who was having condomless sex, who was at risk. He had bipolar disorder and was on lithium chronically and had chronic kidney disease. And so he did not meet the guidelines for Truvada. So we had a very open discussion and he did not feel comfortable with using condoms. He was of that age where condoms weren't really a thing. And so he wanted to practice barebacking. I said, “Okay, if you want to practice barebacking, let's just make sure that you have regularly scheduled visits to screen for STIs. We’ve gotta continue to monitor your renal function and Descovy is actually the option for you.” And so we've been able to keep him on Descovy, keep him HIV negative, and his renal function has remained stable.
Richard: Not every person for whom Descovy might be better will sound like the patient just described. But making the point that there is a time and a place where having these two options for PrEP may be a good thing and increased access for everybody. Having the option to change is empowering like Dr. Pitts’ patient who, because of other medications he took could now have condomless sex, which was his choice and take Descovy safely.
Sam: But not everyone is going to be like Dr. Pitts’ patient. In fact, Damon is someone who hasn't had any poor health side effects from almost a decade on Truvada as PrEP for HIV.
Damon: Everyone keeps asking me, so don't you want to take Descovy? I mean you are over the age of 40 you are in that group of people, slight as it might be, who have seen kidney changes when using Truvada daily. And my answer is “Not yet”. I'm not saying no, and I'm not saying yes because I do not feel like I have adequate information to make the determination yet to know whether Descovy will be safer or not for me personally. I don't feel like the data is there and those safety protocols that I would consult are there. I'm not telling anyone else that they have to do that, but I'm saying for me, that's the right decision.
Sam: Damon is waiting for more information, more data to come out. So he feels like he can make the decision one: himself and two: with stronger data.
Damon: So I'm not telling people like, "Don't use Descovy," but I'm saying, "Please, if you're not in one of those groups that has documented side effects from using Truvada, would it be to your advantage to wait or to get more information before jumping to the newest drug just like the newest iPhone?”
Richard: We've really dug into the side effects for Descovy and Truvada as PrEP. We know that Truvada has more studies on its safety. And as a result is recommended as the first line option Descovy, in turn, is a viable second line option for certain folks who may not be able to get on Truvada or who can't continue due to certain side effects. This is all in the context of continuous PrEP use – meaning you take one pill every day, but there's another place where Truvada beats out Descovy: PrEP on demand.
Sam: Wait, Richard. Is PrEP one of those late night channels I can watch when my parents aren't home?
Gaby: So, what we're talking about is called event driven use, or basically you don't take PrEP daily, but sex knocks at your door and you want to answer. The method is called two, one, one. You take two Truvada pills, 24 hours before the sexual encounter. And then one a day after. And another two days later.
Richard: A 2015 study called IPERGAY – you can't make this shit up – showed that PrEP two, one, one reduced risk of HIV infection by 86% compared to placebo. So we asked Dr. Pitts about Descovy and the two, one, one strategy.
Dr. Pitts: There's no data for PrEP on demand for Descovy
Richard: And then we asked Dr. Blackstock:
Dr. Blackstock: Descovy has not been studied as on demand PrEP. And so people who are interested in taking PrEP using on demand approach, which means, you know, around the before and after, one has sex that should use Truvada as the medication.
Sam: Okay. So that was clear. No need to summarize.
Gaby: The drug is out there. There are some folks that Dr. Blackstock, Damon and Robert agree are going to benefit for daily – emphasis here on daily – use, even while we wait for more data to emerge. But what about the more systems-level impact that having two PrEP options will create?
Sam: The ongoing discussion about discovery and Truvada as PrEP starts with side effects, but it doesn't end there. It's important to mention that the number of PrEP options has broader impact on who might have access to PrEP and how medical providers handle having two drugs.
Richard: There's a lot of evidence that shows how some communities most at risk for getting HIV are not those who have the same amount of access as others, specifically, Black and Latinx men who have sex with men and trans women are less likely to start taking PrEP and less likely to stay on PrEP as compared to their white peers. This is likely due to access because it is often more complicated for folks to get to healthcare providers and then to get those healthcare providers to recommend prevention like PrEP.
Sam: We brought up this issue around disparities and access to Dr. Blackstock to see if she thought having two options would be a way to progress on this issue. At face logic, having a drug become generic would increase financial access to it. And that is predicted to happen for Truvada at the end of 2020
Gaby: But interestingly Dr. Blackstock had a slightly different take:
Dr. Blackstock: I almost feel like it may make things worse potentially because they're a number of populations that are left out of having this potential second option. When people have options, their willingness to try something really increases. And so I think the same thing with PrEP: if we were able to offer potentially two different options or more, that might increase PrEP uptake potentially. And we don't have that for a segment of the population.
Sam: What Dr. Blackstock is saying is that having a second option that is brand name and only approved for men who have sex with men could do very little to increase access to the populations who are more impacted by HIV.
Richard: Allowing healthcare providers, two options for PrEP – one that's okay in people who have vaginas and one that is not yet tested in people who have vaginas – might lead everyone to get confused about how and when to give PrEP to anyone.
Gaby: Even if we put this confusion over prescribing aside, there are other reasons why having Descovy may decrease access to PrEP.
Dr. Blackstock: And I think also the issue is that this could potentially stigmatize Truvada generics, which could potentially also worsen inequities as well. I'm just thinking about people who may be in other parts of the country where Medicaid for instance didn’t expand. And may not have, for instance, access to a brand new medication, but could maybe afford generic medication.
Gaby: Even though there are new options, the potential for misperceptions around them is to quote Damon, having to choose between the newer iPhone and the older one.
Richard: The older one works well and it's fine and it's safe. And we still don't know where the bugs in the software are in the new one, but it's new. So people want it.
Sam: I asked Robert about this and he gave me some interesting context about HIV medication and treatment in general.
Dr. Pitts: Whenever a new drug comes out, it's very exciting. Patients have more options and I know from the HIV realm, when Biktarvy came out, it was an automatic easy switch. Everyone was like, “okay, sounds good”.
Richard: Dr. Pitts is talking about a recently approved HIV treatment that's a three drug regimen and a single pill that was found to be safe and effective and is now one of the mainstays of HIV treatment.
Dr. Pitts: I think Gilead and other companies for those switches helped motivate that and encourage that sort of mentality. Like, “Hey, we have a new drug. It seems better – let's just switch everyone” without any sort of kind of thinking through it
Gaby: But it's also worth noting that what Dr. Pitts is talking about is a very different time in medicine and in HIV history.
Richard: Right. Back in the early days of HIV, when drug development was happening really quickly, it was really important to switch people to the newer medications that came out because they were safer for longer term use. And so providers who were taking care of patients with HIV really partnered and depended on information from drug companies about what was safe and what new studies were coming out. However, in the era of PrEP that might not be so needed.
Gaby: And we should say that Dr. Pitts was on board with this. He also opposed switching PrEP without a discussion with patients.
Dr. Pitts: Providers shouldn't be switching patients from Truvada to Descovy without any sort of conversation. And I know that's happened, unfortunately. But the way we practice at Bellevue is patients ultimately make that decision. And providers can help with that decision, but the patient has to make it. I think PrEP and sexual health is really a gateway into amazing primary care cause once you put someone on PrEP and they accept it, they're going to stay on PrEP. And then you can talk about other needs.
Sam: That echoes something that Damon and Dr. Blackstock had also said, PrEP is more than HIV prevention –
Gaby: – it's been a way to establish meaningful relationships between healthcare providers and patients.
Richard: PrEP is a really powerful form of prevention that really helps people to have autonomy and make decisions about their own sexual health. This new option has broadened the landscape, which for all intents and purposes is a really good thing, but it's really important to maintain this autonomy and make sure that people understand their options so we can make all of the best decisions for people moving forward.
Gaby: This conversation is going to continue. In September, Truvada becomes generic. So these issues we're talking – around access and perceptions of brand versus generic options – all of this may become reality really soon.
Sam: And there's a promising new drug option and injectable for PrEP for HIV that's in the pipeline and has strong data supporting it.
Gaby: And the more options we're going to have, the more important the conversation around choosing the safest and most empowering option for PrEP for HIV is going to become.
Richard: A note for our listeners. In October of 2020, Tenofovir and Emtricitabine in combination became available as a generic version of PrEP. This is will likely be good for access for people who need it overall, but means that the assistance programs from Gilead (the maker of both Truvada and Descovy) will no longer cover co-pays. This is the first time there has ever been a generic form of PrEP! People taking Truvada may notice new co-pays from there insurance and some back and forth between their providers, pharmacies and insurance companies until these processes get ironed out. It shouldn’t be terribly complicated, but persevere. The benefits of PrEP are worth it! Let's give the final word to Damon:
Damon: I know that PrEP medically stands for preexposure prophylaxis for me and for thousands of consumers. It has come to mean proactive, responsible, empowered pleasure. If you're not sure how to talk to your consumers about Descovy or Truvada, consider asking yourself what is the best way I could help the people I care about experience, proactive, responsible, empowered, pleasure, and go from there.
[OUTRO]
Gaby: QHP is a power-sharing project. We place community stories in conversation with health experts to expand autonomy for sexual and gender minorities.
Richard: We would like to thank our guests, Dr. Oni Blackstock, Dr. Robert Pitts and the fabulous Damon Jacobs.
Sam: If you want to engage more with Damon's community work, you can check out his website, www.damonljacobs.com. And we'll also link to his Facebook group PrEP Facts and articles he's written at thebody.com in our show notes.
Gaby: Speaking of show notes, you can check us out www.queerhealthpod.com, where you can find our show notes, resources that we've talked about on the air and more. And we're also on Instagram and Twitter where our handle is @QueerHealthPod. So if you have questions, comments, or feedback, we would absolutely love to hear from you.
Sam: And thank you to Lonnie Ginsburg for composing our theme music.
Richard: Opinions in this podcast are our own and do not represent the opinions of any of our affiliated institutions. And even though we're doctors, please don't use this podcast as medical advice, but instead consult with your own healthcare provider.